The continuing decline in the quality of petroleum feedstocks and the recently enacted regulations limiting the emissions of pollutants have made sulfur and nitrogen removal one of the paramount problems in the refining industry[1-2]. Researchers throughout academy and industry have developed a variety of new hydrotreating catalysts for the ultra⁃deep hydrodesulfurization (HDS),among which the transition metal phosphides showed high activities and were expected to be a new generation of hydrotreating catalysts replacing the traditional metal sulfide catalysts.
A number of metal phosphides (such as MoP[3-5],WP[4,6],Fe2P[7-8],FeP[8-9],CoP[7,10-13],Co2P[4,11-13] and Ni2P[7,13-17]) have been successfully synthesized and used for HDS reactions. Oyama et al[7] prepared SiO2 supported Ni2P,CoP and Fe2P by the temperature⁃programmed reduction (TPR) method,and found that the activity of the phosphides for the HDS reactions followed the order of Ni2P/SiO2>CoP/SiO2>Fe2P/SiO2,while that for the hydrodenitrogenation (HDN) reactions followed the order of CoP/SiO2>Ni2P/SiO2>Fe2P/SiO2. In particular,the Ni2P/SiO2 was shown to be more active (90% conversion of dibenzothiophene (DBT)) than the commercial Ni⁃Mo⁃S/Al2O3 (76% conversion of DBT). In comparison,the CoP/SiO2 exhibited 32% conversion of DBT at the same conditions. Bussell et al[11] found that the HDS activity of thiophene on the CoxPy/SiO2 catalysts prepared by the TPR method was affected by the composition of CoxPy,and the pure phase of CoP/SiO2 showed the highest activity. In addition,Co2P/SiO2 and CoP/SiO2 catalysts were also prepared by TPR method for the hydrodechlorination of chlorobenzene[12]. It was found that the cobalt phosphides were more active than metallic Co,and the activity increased when more P were incorporated for the transformation from Co2P to CoP. Thus,the catalytic activity for the reaction followed the order of CoP/SiO2>Co2P/SiO2>Co/SiO2.
The metal phosphide catalysts reported in literature were mostly obtained by the TPR method. Although this process was simple,the required temperature was relatively high,leading to catalysts with large particles and poor dispersions. Thus,developing metal phosphide catalysts with higher dispersion and more active sites is still highly desirable. Recently,a series of highly dispersed Ni2P catalysts supported on SiO2[18],Al2O3[19-20],MgAlO[19,21] and ZrO2⁃Al2O3[22] were prepared in our lab by a liquid phase phosphidation process using triphenylphosphine (PPh3). The above catalysts possessed small Ni2P particles with diameters of about 6~8 nm,high CO uptakes of about 269~324 μmol·g-1 and thus high activities for the HDS of DBT,HDN of quinoline and hydrogenation of tetralin to decalin. One of the key factors to the above success could be attributed to the usage of highly loaded (60wt%~80wt%),reduced and dispersed Ni catalysts as precursors. In this way,Fe2P/C and FeP/C were also prepared[23]. The FeP/C was found to be active and stable for the HDS of DBT while the Fe2P/C was unstable and converted into Fe0.96S during the hydrotreating reactions. When Al2O3 was used as the support,the dispersion of supported Fe was enhanced and the FeP/Al2O3 with smaller FeP particles (~16 nm) was prepared,which exhibited significantly higher activity than the FeP/C for the HDS reactions[24].
Compared with Ni2P catalysts,the studies on the cobalt phosphides for HDS reactions attracted less attention over the past decades owing to their relatively lower activity for the HDS reactions[1]. In addition,the cobalt phosphide catalysts reported in literature usually possessed large CoP or Co2P particles with small amounts of surface active sites[4,7,10-11]. Previously,we prepared highly loaded Co/SiO2 catalysts (containing 60wt%~80wt% Co) for Fischer⁃Tropsch synthesis[25-26] which exhibited the high reducibility and dispersion of cobalt with small Co particles (11~13 nm) and high H2 uptakes (71~118 μmol·g-1)[26]. In this work,a 60%Co/Al2O3 catalyst was prepared and then phosphided into Co2P/Al2O3 and CoP/Al2O3 catalysts using PPh3 in the liquid phase. Due to the strong interactions of cobalt species with Al2O3,the Co/Al2O3 catalyst possessed highly dispersed cobalt species. A small amount of carbon was added into the Co/Al2O3 in order to promote the reduction of supported cobalt species[26]. A Co9S8/Al2O3 catalyst was also prepared by the sulfidation of the above 60%Co/Al2O3 using CS2 in liquid phase. The activities of these catalysts were compared for the HDS reactions in a model diesel.
1 Experimental
1.1 Preparation of catalysts
The 60%Co/Al2O3 catalyst was prepared by the co⁃precipitation method[24,26]. In order to promote the reduction of cobalt,a resorcinol formaldehyde resin gel (RFG) was added during the precipitation process,which would be converted into carbon (with a theoretical content of 1wt%) during the reduction process. The RFG was prepared as reported previously[26]. Specifically,desired amounts of resorcinol and 37% aqueous formaldehyde (with the molar ratio of 1∶2) were dissolved in 400 mL distilled water and heated at 353 K for 4 h to form a gel in the presence of Na2CO3 as a catalyst. Desired amounts of Co(NO3)2 and Al(NO3)3 were dissolved in 100 mL distilled water to form a solution (Ⅰ). Another solution (Ⅱ) was obtained by dissolving a desired amount of Na2CO3 in 100 mL distilled water. Then,the two solutions (Ⅰ and Ⅱ) were dropwise added into the RFG solution at 353 K under vigorous stirring. The precipitate formed was filtered and washed thoroughly with distilled water. Then,the filter cake was dispersed into 200 mL n⁃butanol and heated at 353 K for 12 h to remove water and then n⁃butanol. The sample was further dried at 393 K overnight.
Cobalt phosphides were prepared in the same way as reported previously[18-22]. Typically,the Co/Al2O3 was loaded into a micro⁃reactor and sandwiched with quartz sands,followed by the reduction in flowing H2 (101.3 kPa,40 mL·min-1) at 773 K for 2 h. Then,the sample was cooled down to 443~593 K,at which a heptane solution containing 2% PPh3 was pumped into the reactor with liquid hourly space velocity (LHSV) of 2 h-1 and H2/oil of 300 (v∶v) for 18~36 h. Finally,the sample was treated in flowing H2 at 673 K for 3 h.
The sulfidation of the Co/Al2O3 was carried out using CS2 in liquid phase[24]. The pre⁃reduced Co/Al2O3 (in H2 at 773 K for 2 h) was sulfided by 2% CS2 in heptane first at 503 K for 2 h and then at 593 K for 4 h,with LHSV of 2 h-1 and H2/oil of 300 (v∶v).
1.2 Characterization of catalysts
The catalysts for characterizations were phosphided or sulfided same way as described above. Then,they were unloaded from the reactor when they were soaked in heptane,which was slowly evaporated at room temperature for 24 h,during which the samples were slowly oxidized by air (passivated). The passivated samples were characterized with different methods.
X⁃ray diffraction (XRD) patterns were collected on a Shimadzu XRD⁃6000 powder diffractometer (Japan) using a Cu Kα radiation. The 2θ scans covered the range of 10°~80° with a scan speed of 6°·min-1.
N2 adsorption⁃desorption measurements were carried out at 77.3 K using a Micromeritics Tristar Ⅱ 3020 autosorption analyzer. The specific surface areas were calculated according to the Brunauer⁃Emmett⁃Teller (BET) equation. Samples were degassed in flowing N2 at 573 K for 3 h before the measurements.
The chemical compositions of catalysts were analyzed by an ARL⁃9800 X⁃ray fluorescence spectrometer (XRF).
The adsorption of O2 was carried out in a home⁃made volumetric apparatus. The catalysts were reduced in H2 at 773 K for 2 h and evacuated for 1 h before the measurements. The adsorption of O2 was performed at 673 K. The uptake of O2 was obtained by extrapolating the coverage of corresponding isotherms to P=0. The degree of reduction (reducibility) was calculated based on the amount of O2 adsorbed and the loading of cobalt (assuming the molar ratio of O∶Co=4∶3)[26-28].
The microcalorimetric adsorption of CO was performed at room temperature (308 K) on a Tian⁃Calvet heat⁃flux microcalorimeter (C⁃80 from Setaram) connected to a gas⁃handling system. A Baratron capacitance manometer was equipped for precise pressure measurements. The passivated samples were re⁃reduced in flowing H2 at 673 K for 2 h and then evacuated at 673 K for 1 h prior to the adsorption measurements.
1.3 Catalytic tests
The hydrotreating reactions of model diesel containing 1.72% DBT (3000 ppm S),0.185% quinoline (200 ppm N),5% tetralin and 0.5% n⁃octane (as an internal standard) in balanced n⁃tetradecane (solvent) were carried out in a fix⁃bed reactor with an inner diameter of 10 mm at 3.1 MPa with weight hourly space velocity (WHSV) of 2 h-1 and H2/oil ratio of 1500 (v∶v). The model diesel was fed into the reactor using a 2ZB⁃1L10 high pressure syringe pump and flowed down through the catalyst bed. For each test,about 1 g catalyst (20~40 mesh) diluted with quartz sands was loaded and sandwiched by two layers of quartz sands (20~40 mesh),and the bed length was about 40 mm. The catalytic activity was measured at 533~633 K. The products were collected after 24 h when the activity was stabilized and analyzed by using GC. Two SE⁃30 capillary columns were connected to a flame ionization detector (FID) and a flame photometric detector (FPD) for the analysis of hydrocarbons and DBT,respectively,while a SE⁃54 capillary column was connected to a nitrogen⁃phosphorus detector (NPD) for the analysis of quinoline.
2 Results and discussion
2.1 Structural and surface properties of catalysts
Table 1 summarizes the textural and structural properties of the catalysts Co/Al2O3,Co2P/Al2O3,CoP/Al2O3 and Co9S8/Al2O3. The phases and particle sizes in the catalysts were derived by XRD (shown shortly below). The reducibility of cobalt was estimated according to the uptake of O2 for the reduced catalyst. The molar ratio of Co∶O was assumed to be 3∶4 since only Co3O4 was detected by XRD for the sample after the O2 titration. The uptake of O2 for the reduced Co/Al2O3 catalyst was about 4030 μmol·g-1,and thus the reduction degree of cobalt in the Co/Al2O3 could be estimated to be 65.7%.
Table 1 Textural and structural properties of the catalysts studied
| Sample | Co/ Al2O3 | Co2P/ Al2O3 | CoP/ Al2O3 | Co9S8/ Al2O3 |
|---|---|---|---|---|
| SBET (m2·g-1) | 161 | 136 | 113 | 120 |
| Pore volume (cm3·g-1) | 0.5 | 0.5 | 0.5 | 0.4 |
| Pore size (nm) | 9 | 15 | 17 | 15 |
| XRD phase | Co | Co2P | CoP | Co9S8 |
| DXRD (nm) | 3.0 | 8.1 | 8.7 | 5.1 |
| O2 uptake (μmol·g-1) | 4030 | – | – | – |
| Reducibility (%) | 65.7 | – | – | – |
| Dispersion (%) | 32 * | – | – | – |
| CO uptake (μmol·g-1) | – | 69 | 76 | 169 |
| CO ads. Heat (kJ·mol-1) | – | 103 | 92 | 107 |
* calculated according to the crystallite size (DXRD).
According to the broadening of Co (111) peak at 44.2° and Scherrer equation,the average crystallite size (DXRD) of Co formed in the reduced Co/Al2O3 was estimated to be about 3 nm. Then the dispersion (D%) of metallic Co could be calculated to be about 32% using the equation: D%=6*14.6*MCo/(ρ*DXRD*NA),in which MCo,ρ and 14.6 are the molar weight (g·mol-1),metal density (g·cm-3) and surface atomic density (atoms·nm-2) of metallic cobalt,respectively,while the NA is the Avogadro constant[4,29]. When the metallic Co was sulfided or phosphided into Co9S8,Co2P and CoP,the crystallite sizes increased to 5.1,8.1 and 8.7 nm,respectively.
Table 1 also gives the information about the specific surface areas and pore parameters. The specific surface area and pore size changed remarkably after the phosphidation and sulfidation of Co/Al2O3,while the pore volumes remained almost unchanged. The specific surface area decreased from 161 m2·g-1 for the reduced Co/Al2O3 to 136,113,and 120 m2·g-1 for the phosphided Co2P/Al2O3 (at 468 K for 18 h),CoP/Al2O3 (at 593 K for 36 h) and sulfided Co9S8/Al2O3,respectively. In contrast,the pore sizes of phosphided and sulfided catalysts were increased (to 15~17 nm) as compared with the reduced catalyst (9 nm).
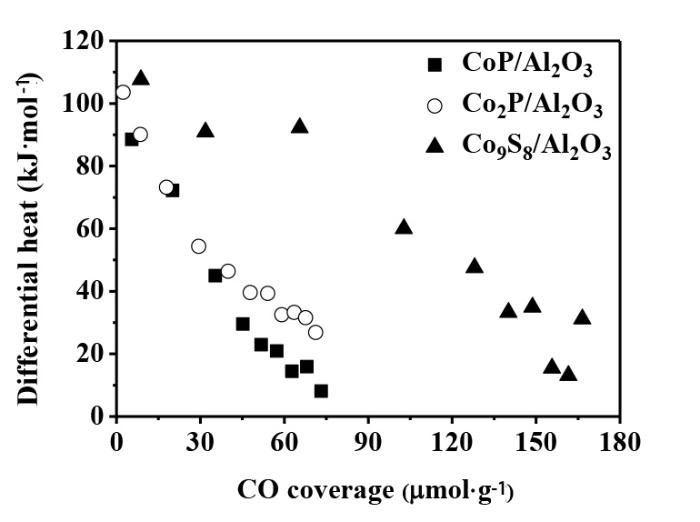
The adsorption of CO is usually used to titrate the surface metal atoms and to probe the number of the active sites for the transition metal phosphide catalysts[1,7]. In particular,the coverage and heat for the adsorption of CO could be obtained simultaneously by the microcalorimetric adsorption measurement[18-22]. The results were shown in Fig.1. The coverages and initial heats for the CO adsorptions on the catalysts Co2P/Al2O3,CoP/Al2O3 and Co9S8/Al2O3 were listed in Table 1. It is seen that the initial heats were measured to be 103,92 and 107 kJ·mol-1 with the saturation coverage of 69,76 and 169 μmol·g-1 on the Co2P/Al2O3,CoP/Al2O3 and Co9S8/Al2O3,respectively. The uptakes of CO on Co2P/Al2O3 and CoP/Al2O3 were significantly higher than those on Co2P/SiO2 (42 μmol·g-1)[30] and CoP/SiO2 (16 μmol·g-1)[7,14] reported in literature,indicating that the Co2P/Al2O3 and CoP/Al2O3 catalysts derived from the liquid phase phosphidation of highly dispersed Co/Al2O3 precursor possessed more surface active sites. The comparable uptakes of CO on the Co2P/Al2O3 and CoP/Al2O3 indicated their similar amounts of surface active sites that might be due to the similar average particle sizes and Co contents[19]. On the other hand,the initial heat for the adsorption of CO on the CoP/Al2O3 was found slightly lower than that on the Co2P/Al2O3,indicating that the higher P content decreased the heat of CO adsorption on Co[14,18]. Fig.1 also showed that the CO uptake on the Co9S8/Al2O3 (169 μmol·g-1) was much higher than those on the Co2P/Al2O3 and CoP/Al2O3,indicating that the Co9S8/Al2O3 possessed more surface active sites,probably due to the significantly higher dispersion of Co9S8 in Co9S8/Al2O3. As shown in Table 1,the particle size of Co9S8 (5.1 nm) were much smaller than those of CoP (8.7 nm) and Co2P (8.1 nm) in the CoP/Al2O3 and Co2P/Al2O3,although they were prepared from the same Co/Al2O3 precursor,but with different treatments.
Fig.1
Fig.1
Differential heat versus coverage for the adsorption of CO at 308 K on the CoP/Al2O3,Co2P/Al2O3 and Co9S8/Al2O3 catalysts ( The samples were re⁃reduced at 673 K in H2 for 2 h and then evacuated at 673 K for 1 h before the adsorptions)
2.2 Control of the CoxP/Al2O3 phases
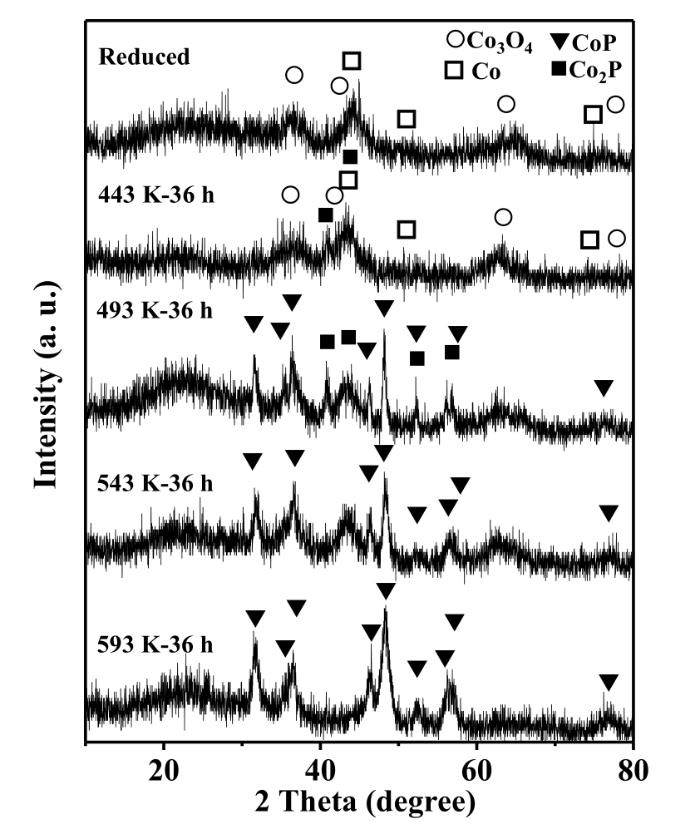
Fig.2 shows the XRD patterns for the samples after the phosphidation of reduced Co/Al2O3 by PPh3 at different temperatures (443~593 K) for 36 h. The weak and broad diffraction peaks around 2θ=44.2°,51.5° and 75.9° characteristic of metallic Co (PDF 15⁃0806) were clearly observed on the reduced Co/Al2O3,indicating that metallic Co was formed and highly dispersed. The weak diffraction peaks at 2θ=36.8°,44.8°,65.2° and 77.3° for Co3O4 (PDF 43⁃1003) detected for the reduced Co/Al2O3 and the sample phosphided at 443 K might be due to the oxidation of surface metallic Co during the passivation.
Fig.2
Fig.2
XRD patterns for the reduced Co/Al2O3 as well as those phosphided by PPh3 at different temperatures for 36 h
The results in Fig.2 showed that the phosphidation temperature affected significantly the phases of CoxP/Al2O3 formed. When the Co/Al2O3 was phosphided at 443 K,most metallic Co remained,while the diffraction peaks at 2θ=40.7°,43.3° attributed to Co2P (PDF 32⁃0306) were also observed,indicating that part of metallic Co was phosphided to Co2P at this temperature. When the Co/Al2O3 was phosphided at 493 K,the diffraction peaks of metallic Co disappeared,and those of Co2P and CoP (PDF 29⁃0497) were clearly seen. When the phosphidation temperature increased to 593 K,only the phase of CoP was observed in the sample.
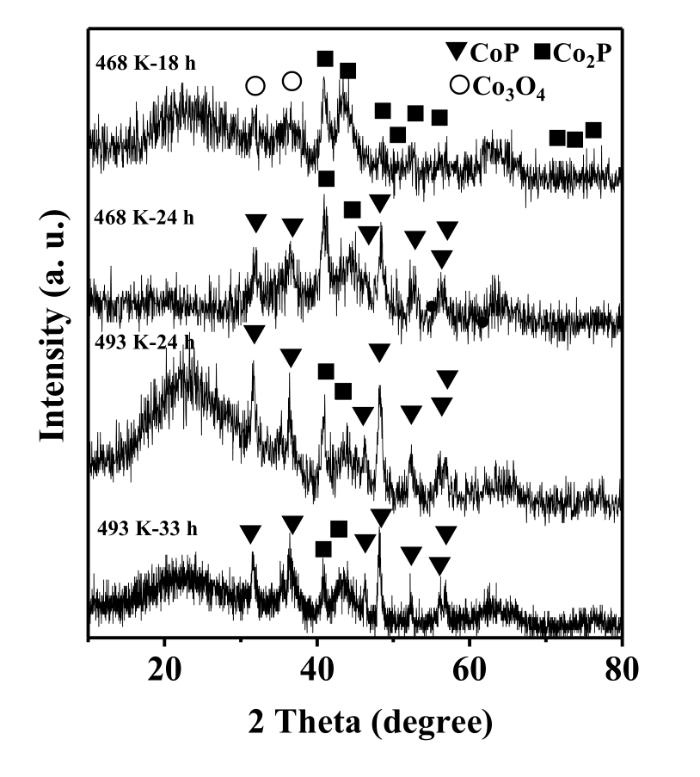
The phosphidation time also affected the phases of CoxP/Al2O3. Fig.3 shows the XRD patterns for the samples after the phosphidation of Co/Al2O3 at 468 and 493 K for different time. When the sample was phosphided at 468 K for 18 h,diffraction peaks of Co2P were observed clearly. The presence of weak peaks for Co3O4 might be due to the oxidation of Co during the passivation. When the phosphidation time prolonged to 24 h at 468 K,CoP appeared in addition to Co2P. Thus,it was difficult to obtain a pure Co2P phase at 468 K. However,it was possible to obtain a catalyst with Co2P as the dominant phase when phosphided at 468 K for 18 h.
Fig. 3
Fig. 3
XRD patterns for the Co/Al2O3 catalyst phosphided by PPh3 at 468 K and 493 K for different time
When the sample was phosphided at 493 K for 24 h,CoP and Co2P were formed. Even if a longer phosphidation time (36 h) was used at this temperature,both CoP and Co2P phases remained.
2.3 Textural and structural properties of used catalysts
The Co2P/Al2O3 and CoP/Al2O3 catalysts obtained from the phosphidation of reduced Co/Al2O3 by PPh3 at 468 K for 18 h and 593 K for 36 h,respectively,were tested for the hydrotreating reactions. The reduced Co/Al2O3 itself and its sulfided sample (Co9S8/Al2O3) were also tested in the same way for comparison.
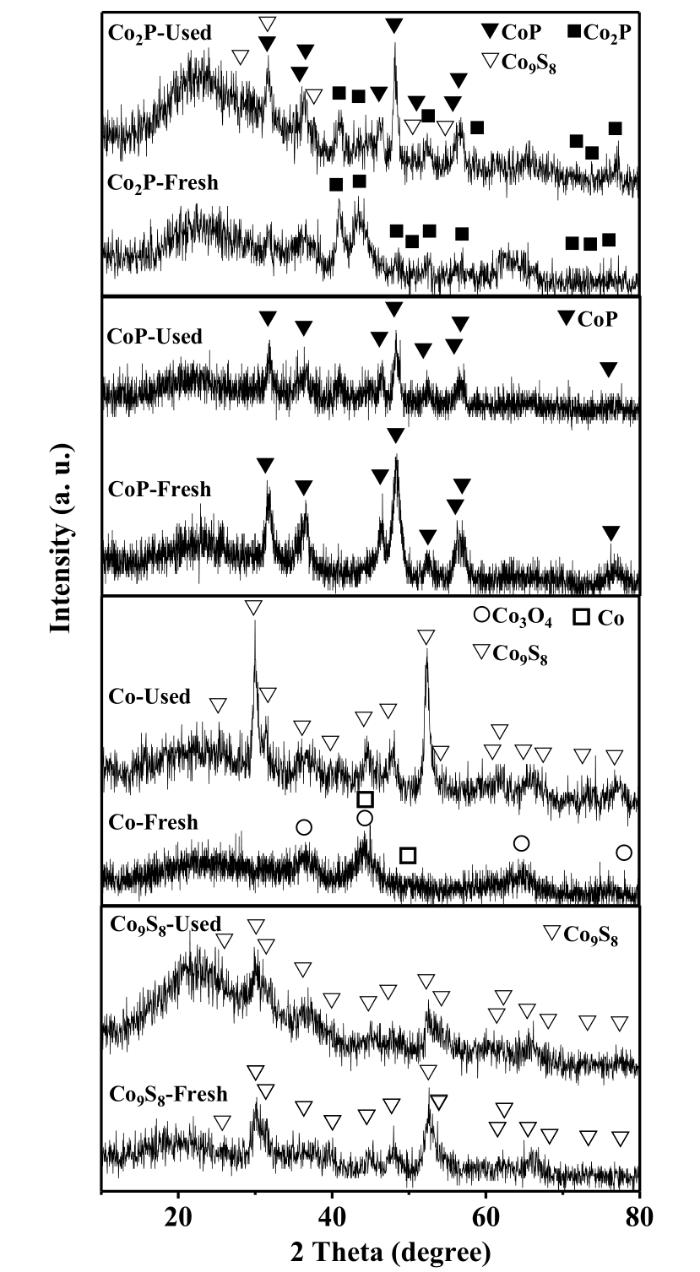
Fig.4 shows the XRD patterns for these catalysts before and after the use for the hydrotreating reactions. Strong diffraction peaks around 2θ=29.8°,31.1°,36.2°,44.8°,47.6° and 51.9° for Co9S8 (PDF 02⁃1459) were clearly seen (without any other phases) in the used Co/Al2O3 after the hydrotreating reactions,indicating that metallic Co could be sulfided into Co9S8,during which the crystallite size was increased from 3 nm for metallic Co to 9 nm for Co9S8.
Fig.4
Fig.4
XRD patterns for the catalysts studied before and after the use for the HDS reactions
When the reduced Co/Al2O3 was sulfided directly by CS2,metallic Co was completely transformed into Co9S8 (5.1 nm). After the use for the HDS reactions,the Co9S8/Al2O3 still exhibited the small size of Co9S8 particles (5.0 nm),indicating the high stability of Co9S8 for the HDS reactions.
The phases CoP and Co9S8 were formed after the Co2P/Al2O3 was used for the HDS reactions. The formation of CoP indicated the disproportionation of Co2P into Co and CoP,and the formed Co might be then converted into Co9S8. According to the broadening of Co2P peak at 40.7° and Scherrer equation,the average sizes of Co2P particles in the fresh and used Co2P/Al2O3 catalysts were estimated to be about 8.1 and 10.5 nm (Table 2),respectively,indicating the growth of Co2P particles during the HDS reactions.
Table 2 Chemical compositions analyzed by XRF and phases determined by XRD for the catalysts before and after the use for the HDS reactions.
| Catalysts | Co (wt%) | P (wt%) | S (wt%) | P/Co (atom ratio) | S/Co (atom ratio) | Phases | Particle size (nm) |
|---|---|---|---|---|---|---|---|
| Co/Al2O3 | 56.9 | - | - | - | Co | 3 | |
| Used Co/Al2O3 | 49.4 | - | 13.1 | - | 0.49 | Co9S8 | 9 |
| Co9S8/Al2O3 | 44.0 | 20.4 | 0.86 | Co9S8 | 5.1 | ||
| Used Co9S8/Al2O3 | 43.2 | - | 20.8 | - | 0.89 | Co9S8 | 5.0 |
| Co2P/Al2O3 | 52.4 | 17.2 | - | 0.37 | Co2P | 8.1 | |
| Used Co2P/Al2O3 | 42.7 | 15.0 | 6.8 | 0.39 | 0.16 | CoP,Co2P,Co9S8 | 10.5 (Co2P) |
| CoP/Al2O3 | 41.1 | 24.0 | - | 1.11 | CoP | 8.7 | |
| Used CoP/Al2O3 | 40.2 | 20.0 | 4.1 | 0.90 | 0.19 | CoP | 11.2 |
In contrast,the CoP phase did not change after the HDS reactions,indicating the high stability of this phase. However,according to the broadening of CoP peak at 48.1° and Scherrer equation,the average sizes of CoP particles in the fresh and used CoP/Al2O3 were estimated to be about 8.7 and 11.2 nm (Table 2),respectively,indicating the growth of CoP particles during the HDS reactions.
Table 2 summarizes the chemical compositions of the fresh and used catalysts. The used Co/Al2O3 contained about 13.1% S due to the formation of Co9S8 during the HDS of DBT. The fresh and used Co9S8/Al2O3 had the S/Co atomic ratios of 0.86 and 0.89,respectively,close to the stoichiometric ratio of 0.89 in Co9S8,indicating again the high stability of Co9S8 for the HDS reactions.
The fresh CoP/Al2O3 exhibited the P/Co atomic ratio of 1.11,close to the stoichiometric ratio of 1 for CoP. However,the fresh Co2P/Al2O3 possessed the P/Co atomic ratio of 0.37,lower than the stoichiometric ratio of 0.5 for Co2P,probably owing to the presence of some un⁃phosphided cobalt species (Fig.3). After the hydrotreating reactions,the P/Co atomic ratios in the used CoP/Al2O3 and Co2P/Al2O3 changed little. However,S was detected by XRF in the used CoP/Al2O3 and Co2P/Al2O3. Specifically,the used CoP/Al2O3 and Co2P/Al2O3 contained about 4.1% and 6.8% S,indicating that CoP was significantly more resistant to S poisoning than Co2P,which was also observed by Bussell et al[11]. In fact,Co9S8 was detected by XRD in the used Co2P/Al2O3,while no any other phases were observed in the used CoP/Al2O3.
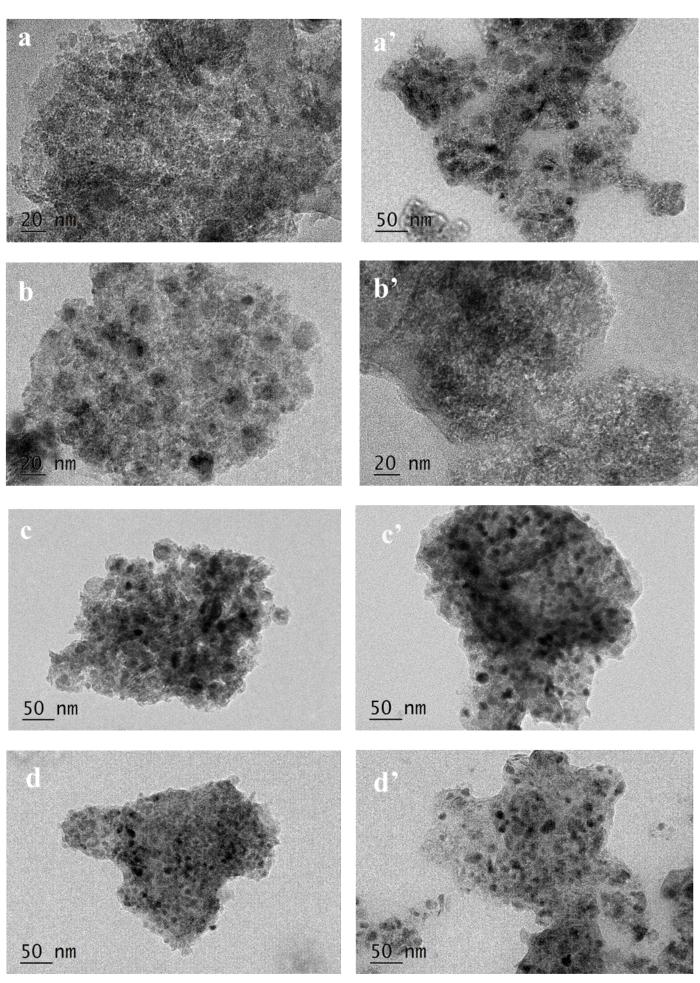
Fig.5 shows the TEM images of fresh and used catalysts. It can be seen that metallic Co particles were well dispersed in the reduced Co/Al2O3. The statistically averaged particle size of Co in the fresh (Fig.5a) and that of Co9S8 in the used Co/Al2O3 (Fig.5a') were about 2.5 and 10 nm,respectively,consistent with those estimated by the Scherrer equation (3 and 9 nm,respectively).
Fig.5
Fig.5
TEM images of the fresh Co/Al2O3 (a),Co9S8/Al2O3 (b),Co2P/Al2O3 (c) and CoP/Al2O3 (d),as well as the used Co/Al2O3 (a'),Co9S8/Al2O3 (b'),Co2P/Al2O3 (c') and CoP/Al2O3 (d') catalysts for the hydrotreating reactionsReaction conditions: T=533~633 K,P=3.1 MPa,WHSV=2 h-1 and H2∶oil=1500∶1 (v∶v)
The TEM images of fresh and used Co2P/Al2O3 are shown in Fig.5c and Fig.5c'. The average particle sizes in the fresh and used catalysts were estimated to be about 8.8 and 11 nm,respectively,close to those (8.1 and 10.5 nm) estimated by XRD,though some fresh Co2P were converted into other phases after the use. The TEM images (Fig.5d and Fig.5d') also showed that the CoP particles in the CoP/Al2O3 grew during the HDS reactions,as already evidenced by XRD.
2.4 Hydrotreating activities of catalysts
Co usually acts as a promoter in Co⁃Mo⁃S catalysts for the hydrotreating reactions in industry[2,31]. However,Co itself was also active for the HDS reactions of thiophene and DBT[32-36]. In an earlier work[37],we prepared a Co⁃O⁃Si complex material with a high specific surface area (560 m2·g-1) at a high Co loading (~34wt%). After sulfidation,the material exhibited a high activity in HDS of thiophene (the conversion of thiophene reached 99.4% at 573 K and 1.5 MPa). In this work,the highly dispersed Co/Al2O3 was sulfided by CS2 to obtain a cobalt sulfide catalyst and compared with metallic Co and cobalt phosphide catalysts for the HDS of DBT.
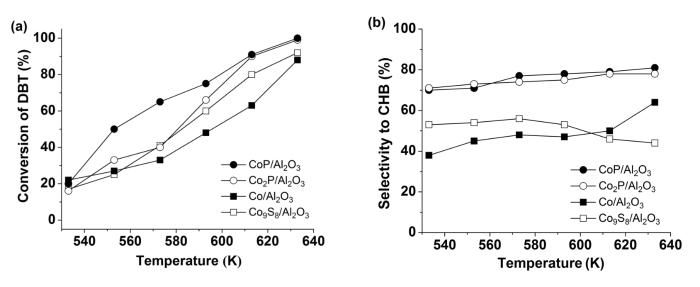
Fig.6a shows the catalytic activities of the catalysts for the HDS of DBT. The conversion of DBT increased with temperature (533~633 K). At 573 K,the conversion of DBT reached 40% and 65%,respectively,over the Co2P/Al2O3 and CoP/Al2O3. Apparently,the CoP/Al2O3 was significantly more active than the Co2P/Al2O3 for the HDS of DBT. At 633 K,the conversion of DBT reached 99% and 100%,respectively,over the Co2P/Al2O3 and CoP/Al2O3.
Fig.6
Fig.6
Conversion of DBT (a) and selectivity to cyclohexylbenzene (CHB) (b) versus temperature on the catalystsOther reaction conditions: P=3.1 MPa,WHSV=2 h-1 and H2:oil=1500∶1 (v:v)
The Co/Al2O3 and Co9S8/Al2O3 exhibited the similar activities to the Co2P/Al2O3 at 533~573 K for the HDS of DBT. However,at 593 K,the conversion of DBT increased to 48%,60% and 66%,respectively,over the Co/Al2O3,Co9S8/Al2O3 and Co2P/Al2O3. Thus,the activities of these catalysts for the HDS of DBT followed the order of CoP/Al2O3>Co2P/Al2O3>Co9S8/Al2O3>Co/Al2O3.
The HDS of DBT usually undergoes through two pathways. One is the direct desulfurization pathway (DDS) with the formation of biphenyl (BP) as the desulfurization product,while another is the indirect hydrodesulfurization pathway with the formation of cyclohexylbenzene (CHB) as the desulfurization product (the desulfurization occurs only after an aromatic ring in DBT is hydrogenated). Fig.6b shows the selectivity to CHB at different temperatures. Apparently,the selectivities to CHB on the CoP/Al2O3 and Co2P/Al2O3 were always significantly higher than those on the Co/Al2O3 and Co9S8/Al2O3. For example,at 613 K,the selectivities to CHB on CoP/Al2O3 and Co2P/Al2O3 were 79% and 78%,while those on Co/Al2O3 and Co9S8/Al2O3 were 50% and 46%,respectively. Since the selectivity to CHB (>70%) was always higher than that to BP on the CoP/Al2O3 and Co2P/Al2O3,the CoP/Al2O3 and Co2P/Al2O3 seemed more active for the hydrogenation of aromatic rings in DBT.
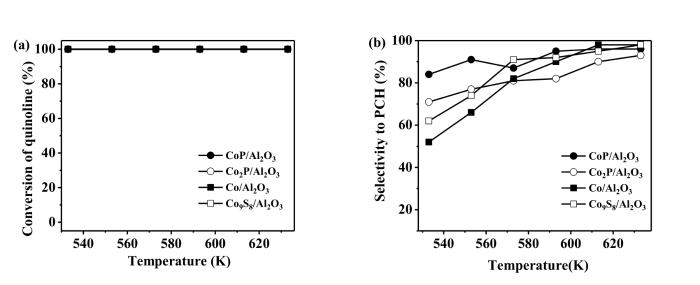
Fig.7a shows the results for the conversion of quinoline at different temperatures. The conversion of quinoline reached 100% on the four catalysts at 533~633 K. Propylbenzene (PB) and propylcyclohexane (PCH) were the products of HDN of quinoline. The former is the product of hydrodenitrogenation without the hydrogenation of another aromatic ring. The latter involves not only the denitrogenation,but also the hydrogenation of another aromatic ring in quinoline. Fig.7b shows the selectivity to PCH at different temperatures. With the increase of temperature,the selectivity to PCH increased on all the four catalysts. At 533 K,the selectivity to PCH on different catalysts followed the order of CoP/Al2O3 (84%) > Co2P/Al2O3 (71%) > Co9S8/Al2O3 (62%) > Co/Al2O3 (52%). Thus,the Co2P and CoP seemed more active than Co and Co9S8 for the hydrogenation of aromatic rings in quinoline at the low reaction temperatures,consistent with the results for the HDS of DBT.
Fig.7
Fig.7
Conversion of quinloine (a) and selectivity to propylcyclohexane (PCH) (b) on the catalystsOther reaction conditions: P=3.1 MPa,WHSV=2 h-1 and H2∶oil=1500∶1 (v∶v)
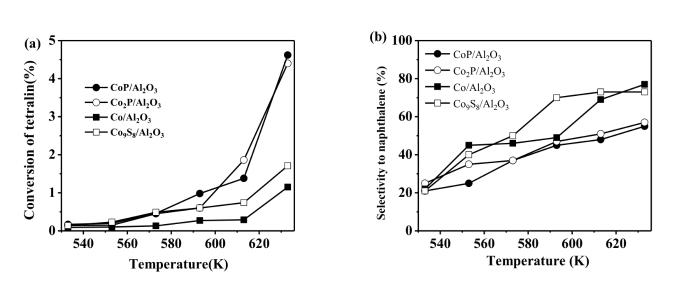
Aromatic hydrocarbons with multi⁃rings have low cetane numbers,and their contents must be reduced to enhance the quality of diesel fuels. In this work,the hydrogenation of tetralin was used to probe the activities of catalysts for the hydrogenation of hydrocarbons with multiple aromatic rings. It can be seen from Fig.8a that the conversions of tetralin were low on all the catalysts. However,the conversions of tetralin were higher on the Co2P/Al2O3 and CoP/Al2O3 than on the Co/Al2O3 and Co9S8/Al2O3. For example,the conversions of tetralin on the CoP/Al2O3 and Co2P/Al2O3 were 4.4% and 4.6%,while those on the Co/Al2O3 and Co9S8/Al2O3 were only 1.1% and 1.7% at 633 K,respectively.
Fig.8
Fig.8
Conversion of tetralin (a) and selectivity to naphthalene (b) on the catalystsOther reaction conditions: P=3.1 MPa,WHSV=2 h-1 and H2∶oil=1500∶1 (v∶v)
Tetralin might be hydrogenated to decalin or dehydrogenated to naphthalene. Fig.8b shows the selectivity to naphthalene over the catalysts at different temperatures. With the increase of temperature,the selectivity to naphthalene increased over all the catalysts. However,the selectivities to naphthalene (dehydrogenation) on the Co/Al2O3 and Co9S8/Al2O3 were significantly higher than those on the Co2P/Al2O3 and CoP/Al2O3,indicating that Co2P and CoP were more active than Co and Co9S8 for the hydrogenation reactions.
3 Conclusions
A 60%Co/Al2O3 catalyst was prepared and reduced. Metallic Co formed was highly dispersed with small Co particles (~3 nm),which could be phosphided to Co2P and CoP by using PPh3 in liquid phase.
The reduced Co/Al2O3 could be phosphided into Co2P and CoP by monitoring the phosphidation temperature and time. Specifically,metallic Co was phosphided mainly into Co2P at 468 K for 18 h,and purely into CoP at 593 K for 36 h. For comparison,metallic Co was also sulfided into Co9S8 by CS2 at 583 K for 4 h.
The CoP/Al2O3 exhibited the significantly higher activity than all the other catalysts (freshly Co2P/Al2O3,Co9S8/Al2O3 and Co/Al2O3) studied in this work for the HDS of DBT. In addition,the phase of CoP was stable and it did not change during the HDS reactions.
The phase of Co2P was not stable since a large amount of Co2P changed into CoP and Co9S8 during the HDS reactions,leading to the decreased activity of this catalyst for the HDS reactions.
Metallic Co in the reduced Co/Al2O3 (~3 nm) was completely converted into Co9S8 with significantly increased particle size (~9 nm) during the HDS reaction. In contrast,the phase of Co9S8 and its particles (~5 nm) in the pre⁃sulfided Co9S8/Al2O3 were highly stable and quite active for the HDS reactions.
Reference
Transition metal phosphide hydroprocessing catalysts:A review
Desulfurization of 2⁃phenylcyclohexanethiol over transition⁃metal phosphides
Synthesis and characterization of silica⁃supported transition⁃metal phosphides as HDN catalysts
Characterization of silica⁃supported molybdenum and tungsten phosphide hydroprocessing catalysts by 31P nuclear magnetic resonance spectroscopy
Synthesis and activity of a new catalyst for hydroprocessing:Tungsten phosphide
Synthesis,characterization,and hydrotreating activity of several iron group transition metal phosphides
Control of Phase in Phosphide Nanoparticles Produced by Metal Nanoparticle Transformation:Fe2P and FeP
A solvothermal synthesis of ultra⁃fine iron phosphide
Gas phase catalytic hydrodechlorination of chlorobenzene over cobalt phosphide catalysts with different P contents
Hydrodesulfurization of dibenzothiophene and its hydrogenated intermediates over bulk CoP and Co2P catalysts with stoichiometric P/Co ratios
Thiophene hydrodesulfurization over supported nickel phosphide catalysts
Novel catalysts for advanced hydroprocessing:Transition metal phosphides
Synthesis of nickel phosphide nano⁃particles in a eutectic mixture for hydrotreating reactions
Effect of supports on the supported Ni2P catalysts prepared by the phosphidation using triphenylphosphine in liquid phase
Effect of loading on the Ni2P/Al2O3 catalysts for the hydrotreating reactions
Optimization of MgO/Al2O3 ratio for the maximization of active site densities in the Ni2P/MgAlO catalysts for the hydrotreating reactions
Effect of ZrO2 in Ni2P/ZrO2–Al2O3 catalysts on hydrotreating reactions
Preparation of Fe2P and FeP catalysts for the hydrotreating reactions
Preparation of Fe2P/Al2O3 and FeP/Al2O3 catalysts for the hydrotreating reactions
Microcalorimetric adsorption studies of highly loaded Co–ZrO2/SiO2 catalysts for Fischer–Tropsch synthesis
Effect of Resorcinol Formaldehyde Resin Gel on the Preparation of Co/SiO2 Catalysts for Fischer⁃Tropsch Synthesis
The stoichiometries of H2 and CO adsorptions on cobalt:Effects of support and preparation
Microcalorimetric studies of H2 and CO on Co/γ⁃Al2O3 catalysts for Fischer–Tropsch synthesis
Study of Pt⁃promoted cobalt CO hydrogenation catalysts
A review of deep hydrodesulfurization catalysis
The role of Co in sulphidised Co–Mo hydrodesulphurisation catalysts supported on carbon and alumina
HDS,HDN and HYD activities and temperature⁃programmed reduction of unsupported transition metal sulfides
Enhanced HDS and HYD activity of sulfide Co⁃PMo catalyst supported on alumina and structured mesoporous silica composite
Synthesis of high⁃surface⁃area Co⁃O⁃Si complex oxide for skeletal isomerization of 1⁃hexene and hydrodesulfurization of thiophene